Keep an eye on the host - January 13, 2022
Disease-Modifying Anti-Rheumatic Drugs (DMARDs) are therapeutic agents that impact RA activity and have been described to impede or arrest structural joint damage. Periodontitis and RA share many similarities in pathophysiology and clinical progression, and these common proinflammatory and tissue-damaging networks involved in RA and periodontitis have raised the issue of DMARDs’ impact on periodontal inflammation.
------
Rheumatoid arthritis (RA) is a chronic autoimmune disease affecting 0.1–2.0% of the population worldwide. It arises more frequently in females, and it is predominantly observed in the elderly, peaking at 65–74 years of age. It primarily manifests as chronic inflammatory arthropathy, leading to cartilage destruction, bone erosions and subsequent impaired joint function. RA is a heterogeneous disease with variable clinical phenotypes and course of progression. Over the last decades, findings that came to light confirmed that irreversible joint damage and deformities could already occur within the first few months of disease onset. Consequently, early diagnosis and appropriate treatment are now recommended, preferably even before any deformity is identified, to slow disease progression and alter its course (“put the disease in remission”).
Over the years, treatment for RA has changed profoundly, evolving from the approach of providing symptomatic relief (e.g. analgesics and NSAIDs) to the application of therapeutic agents that impact disease activity and have been described to impede or arrest structural joint damage. The latter fall under the umbrella term Disease-Modifying Anti-Rheumatic Drugs (DMARDs). There are two major classes of DMARDs, namely synthetic (s-) and biological (b-) DMARDs or biologics. Synthetic DMARDs can be further divided into conventional (csDMARDs) and targeted synthetic DMARDs (tsDMARDs). The former represent the oldest class of agents and include standard systemic medications that suppress the immune system more broadly than biological or tsDMARDs. Their use has evolved empirically, and their modes of action are not fully elucidated. Gold salts were the earliest form of csDMARDs, available since the 1920s. In the following years, new csDMARDs became available (sulfasalazine in the 1940s and hydroxychloroquine in the 1950s). In the 1980s, methotrexate (MTX), a drug commonly used to treat psoriasis, was proven to be safe and well-tolerated in RA. Therefore, in 1988 MTX received the US Food and Drug Administration (FDA) approval for the medical management of RA, and by the 1990s, it had become its first-line therapy.
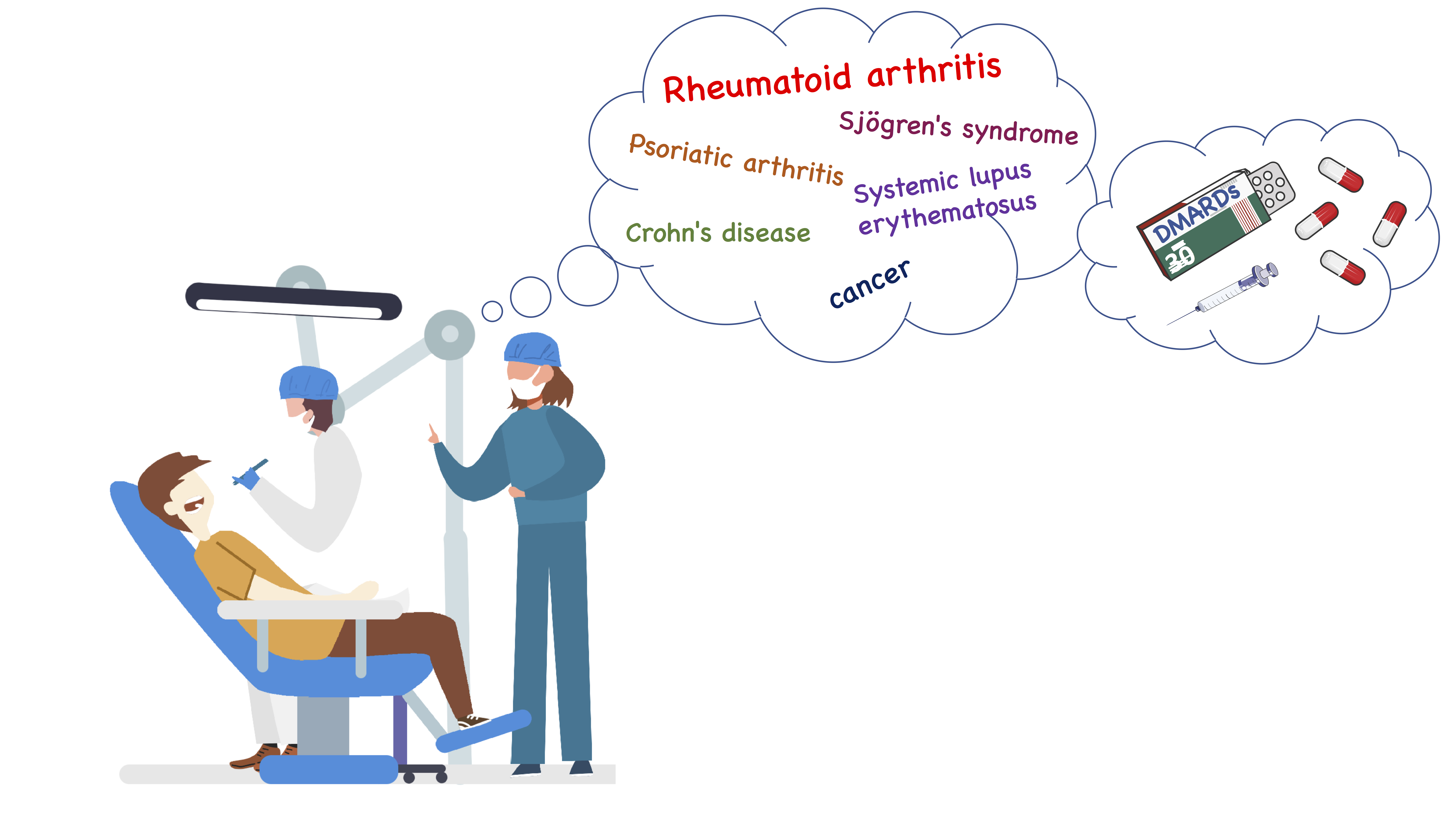
The development of bDMARDs in the 1990s offered further opportunities to treat the disease. In contrast to csDMARDs, biologics and tsDMARDs have been developed to modulate specific targets in the inflammation process. These agents include monoclonal antibodies and genetically engineered proteins directed against cytokines or cell-surface molecules. The earliest agents inhibited the biological activity of TNF-a, a cytokine known to perpetuate the inflammatory response in RA, leading to synovial proliferation and bone destruction. Following the introduction of the first TNF inhibitors (etanercept, infliximab, adalimumab etc.), bDMARDs targeting other components of the immune response known to be involved in the pathogenesis of RA, have been approved for clinical use (rituximab, tocilizumab etc.). Usually, bDMARDs are prescribed in conjunction with MTX. However, more than a third of patients are intolerant of MTX, so approximately 30% of patients in clinical practice are treated with bDMARD monotherapy. When considering the overall use of DMARDs, it should be mentioned that these drugs are not entirely exclusive to RA, as they may also be used to treat other autoimmune diseases such as psoriasis, systemic lupus erythematosus (SLE), Sjögren’s syndrome, Crohn’s disease, and some types of cancer.
This raises some questions: How common is the use of DMARDs in the overall population and within the pool of periodontal patients? An epidemiological study using data from a large German health insurance database found an increase in the use of DMARDs between 2004 and 2011. In fact, the use of cDMARDs, increased from 6.530/00 in 2004 to 8.930/00 in 2011, whereas the use of bDMARDs presented a four-fold increase from 0.350/00 in 2004 to 1.540/00 in 2011 with a substantially higher prescription prevalence in women aged 50 to 79 years. MTX was the most frequently prescribed cDMARD, followed by azathioprine and sulfasalazine. Accordingly, the highest prescription prevalence in bDMARDs was observed for adalimumab, followed by etanercept and rituximab.
Periodontitis and RA share many similarities in pathophysiology and clinical progression, and these common proinflammatory and tissue-damaging networks involved in RA and periodontitis have raised the issue of DMARDs’ impact on periodontal inflammation. Since no study has evaluated the prevalence of DMARDs among periodontal patients until now, one way to approach this topic is by asking how often do we meet patients diagnosed with RA who also require periodontal treatment, assuming that these patients might already have received treatment with some DMARDs. More than two decades ago, a group of researchers from the University of Queensland’s School of Dentistry in Australia performed a study to explore this prevalence. The researchers analysed the data obtained from self-reported health questionnaires and dental records of 1412 individuals who had been either referred for advanced periodontal care (test group) or attended a clinic for general dental treatment (other than referred periodontal treatment). They found that 4 out of 100 patients in the periodontitis group had RA (3.95%), and this number was significantly greater than that found in the general group (GP) (0.66%). These findings describe that DMARDS-medicated patients are not uncommonly found among those seeking periodontal help. The study also described that patients with RA were more likely to suffer from advanced bone loss (62.5%) than those without RA (43.8%), indicating that when RA patients seek our help, they may require more challenging treatment due to the advanced stage of periodontitis.
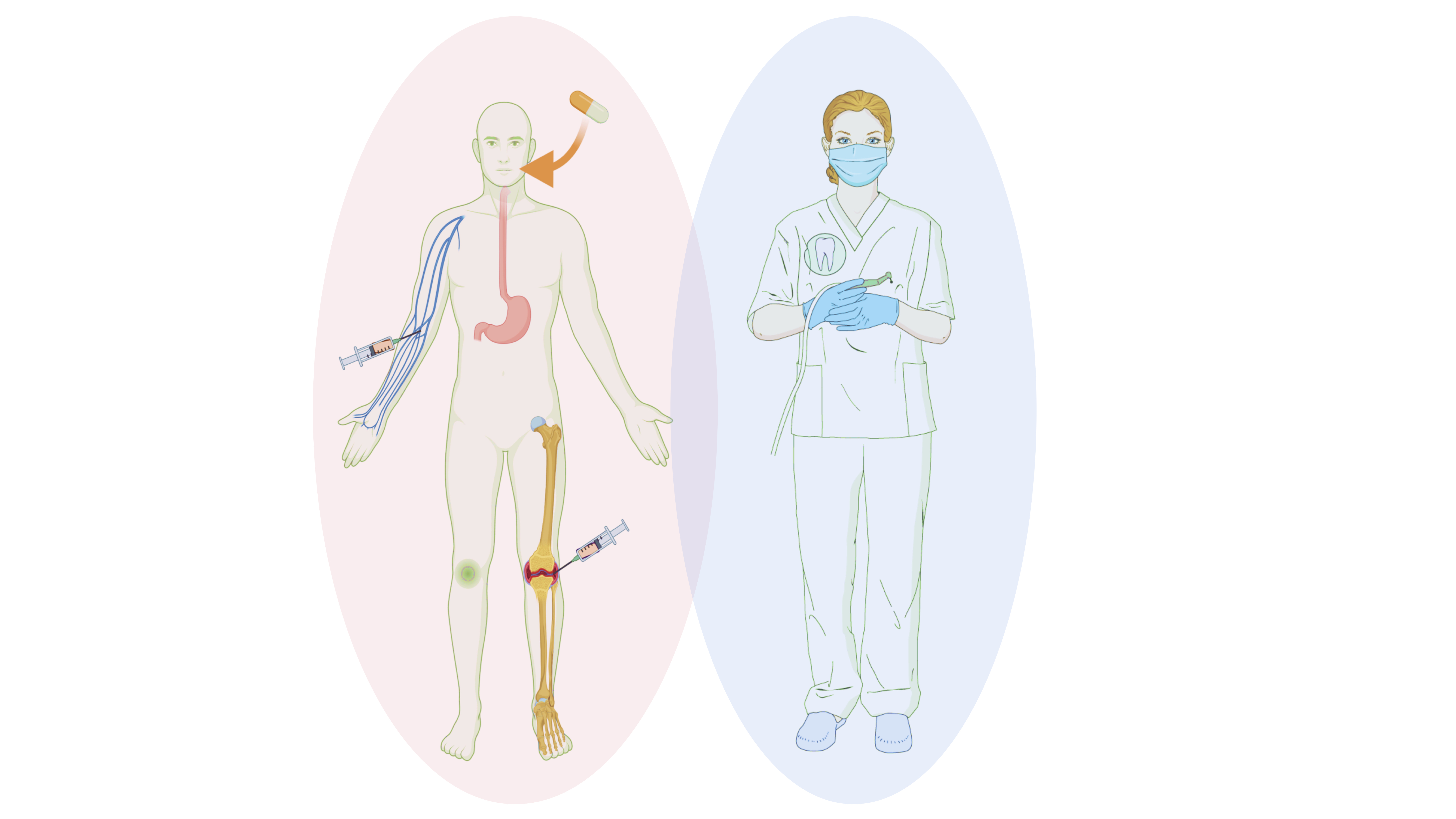
To date, most evidence on the impact of these synthetic and biologic immunomodulators on periodontitis are small-scale cross-sectional clinical studies, evaluating the periodontal status in patients undergoing treatment for RA. Some of these studies report that the combination of csDMARDs (methotrexate with leflunomide) is associated with higher clinical attachment loss in patients with established RA, whereas other investigations, including RA patients with low periodontal inflammation, reported no significant differences in the periodontal parameters of patients under DMARDs vs those who did not receive such drugs in the absence of any additional periodontal therapy. In contrast, a recent prospective study by Jung and colleagues, investigating the adjunctive use of csDMARDs, such as methotrexate, hydroxychloroquine, and sulfasalazine, on the response to non-surgical periodontal treatment, reported an additional benefit in the DMARDs group.
In the case of biologics, the data remain somewhat inconsistent. A small body of evidence demonstrated the significant benefit of TNF-α inhibitors (etanercept, infliximab, adalimumab etc.) on periodontal clinical parameters when delivered alone or in combination with periodontal treatment in patients with RA, while other studies described aggravated gingival inflammation with higher BOP. It was recently reported that the duration of anti-TNF therapy might influence periodontal clinical parameters since a trend for additional clinical benefit was described when these drugs were administered for periods of 6 weeks to 6 months. On the contrary, intake for >6 months was associated with higher gingival index and BOP values. This could be due to decreased compliance or secondary loss of response possibly related to the development of anti-drug antibodies, a frequent finding during treatment with bDMARDs. However, treatment with other biologics such as tocilizumab (an interleukin-6 receptor blocker) without additional periodontal treatment and/or oral hygiene instructions resulted in a small improvement in PPD and clinical attachment level (CAL) after 6 months. In addition, the percentage of sites with BOP, PPD ≥4mm, and CAL ≥4mm decreased significantly after 6 months of tocilizumab. Similarly, patients who received rituximab (a B-cell blocker) for other diseases presented with less severe periodontitis after 6 months.
Apart from the -mabs, the -nibs (baricitinib, tofacitinib etc..) have lately joined the family of DMARDs. These are Janus kinase (JAK) inhibitors that represent a new class of oral medications recently approved for the treatment of RA. 24 weeks of therapy with baricitinib without any additional periodontal therapy and/or oral hygiene instructions resulted in a significant reduction in PPD, percentage of sites with PPD ≥4 mm, and sites with BOP, indicating a clinical benefit.
All this data are based on a limited number of patients who have been primarily selected on the basis of being diagnosed with RA and secondarily due to their periodontal aggravated status. As a result, these study populations are probably rather heterogeneous in terms of the periodontal disease severity and make any conclusion drawing quite challenging. Finally, one should bear in mind that none of these drugs is “intelligent” enough to be able to distinguish the factor/cytokine involved in disease pathogenesis from that involved in immune defence against external agents, and thereby lies the point of departure for many significant side effects, including a high risk of infection, especially after surgical operations such as implant therapy. In medical sciences, it is the cost/benefit ratio that settles the issue whether we are dealing with a “friend” or a “foe”, and this has to be established by prioritising the most life-threatening conditions in our clinical considerations. The findings from all these studies can, nevertheless, serve as a valuable indication of what we as periodontists may expect in terms of clinical status when patients using -mabs and -nibs cross the door of our clinic. As Aristophanes, the well-known comic playwright of ancient Athens wrote in his comedy entitled „Birds“: “A man may learn wisdom even from a foe”.
Literature and suggested reads
Atzeni, F., & Sarzi-Puttini, P. (2019). The therapeutic journey of biologic agents: There will be an end?. Pharmacological Research, 147, 104340. https://doi.org/10.1016/j.phrs.2019.104340
Ancuța, C., Pomîrleanu, C., Mihailov, C., Chirieac, R., Ancuța, E., Iordache, C., Bran, C., & Țănculescu, O. (2020). Efficacy of baricitinib on periodontal inflammation in patients with rheumatoid arthritis. Joint Bone Spine, 87(3), 235–239. https://doi.org/10.1016/j.jbspin.2019.12.003
Äyräväinen, L., Leirisalo-Repo, M., Kuuliala, A., Ahola, K., Koivuniemi, R., Meurman, J. H., & Heikkinen, A. M. (2017). Periodontitis in early and chronic rheumatoid arthritis: a prospective follow-up study in Finnish population. BMJ Open, 7(1), e011916. https://doi.org/10.1136/bmjopen-2016-011916
Balta, M. G., Papathanasiou, E., Blix, I. J., & Van Dyke, T. E. (2021). Host Modulation and Treatment of Periodontal Disease. Journal of Dental Research, 100(8), 798–809. https://doi.org/10.1177/0022034521995157
Mercado, F., Marshall, R. I., Klestov, A. C., & Bartold, P. M. (2000). Is there a relationship between rheumatoid arthritis and periodontal disease?. Journal of Clinical Periodontology, 27(4), 267–272. https://doi.org/10.1034/j.1600-051x.2000.027004267.x
Benjamin O, Bansal P, Goyal A, et al. Disease Modifying Anti-Rheumatic Drugs (DMARD) [Updated 2021 Jul 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507863/
Cillo, J. E., Jr, & Barbosa, N. (2019). Adalimumab-Related Dental Implant Infection. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons, 77(6), 1165–1169. https://doi.org/10.1016/j.joms.2019.01.033
Fassmer, A. M., Garbe, E., & Schmedt, N. (2016). Frequency and trends of disease-modifying antirheumatic drug (DMARD) use in Germany. Pharmacology Research & Perspectives, 4(5), e00254. https://doi.org/10.1002/prp2.254
Jung, G. U., Han, J. Y., Hwang, K. G., Park, C. J., Stathopoulou, P. G., & Fiorellini, J. P. (2018). Effects of Conventional Synthetic Disease-Modifying Antirheumatic Drugs on Response to Periodontal Treatment in Patients with Rheumatoid Arthritis. BioMed Research International, 2018, 1465402. https://doi.org/10.1155/2018/1465402
Pers, J. O., Saraux, A., Pierre, R., & Youinou, P. (2008). Anti-TNF-alpha immunotherapy is associated with increased gingival inflammation without clinical attachment loss in subjects with rheumatoid arthritis. Journal of Periodontology, 79(9), 1645–1651. https://doi.org/10.1902/jop.2008.070616
Zamri, F., & de Vries, T. J. (2020). Use of TNF Inhibitors in Rheumatoid Arthritis and Implications for the Periodontal Status: For the Benefit of Both?. Frontiers in Iimmunology, 11, 591365. https://doi.org/10.3389/fimmu.2020.591365
Ziebolz, D., Rupprecht, A., Schmickler, J., Bothmann, L., Krämer, J., Patschan, D., Müller, G. A., Mausberg, R. F., Schmidt, J., Schmalz, G., & Patschan, S. (2018). Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: Results from a cross-sectional study. Journal of Periodontology, 89(11), 1310–1317. https://doi.org/10.1002/JPER.17-0616
