A young Periodontist's contribution - February 21, 2020
The enamel matrix derivative (EMD) is a widely used biological agent that can improve the healing and regeneration of the periodontal wound. EMD shows anti-inflammatory and angiogenic properties and can promote both fibroblast proliferation and osteoblast differentiation. Recent evidence has shown that an intensive non-surgical periodontal therapy in association with EMD can produce more significant improvements in the clinical periodontal parameters and a lower perturbation of systemic inflammation when compared to the non-surgical treatment alone.
Dissertation title: “The impact of enamel matrix derivative on systemic inflammation markers after non surgical periodontal therapy: a randomized clinical trial”
Dissertation Author: Laura Bettini
University: University of Pisa, Italy
Supervisor: Professor F. Graziani
EGR (EMD Guided Regeneration)
The Enamel Matrix Derivative (EMD) is a derivative of the extracellular matrix from porcine tooth buds, mainly composed of amelogenins immersed in a viscous aqueous solution, known for promoting periodontal regeneration.
The Hertwig epithelial root sheath (HERS) of developing teeth deposit an extracellular matrix similar to the enamel matrix secreted by mature ameloblasts, and the acellular cement contains proteins similar to enamel matrix proteins (EMPs). The introduction of EMD in periodontal regeneration was based on the apparent involvement of EMPs in the formation of acellular cement and of supporting tissues of the tooth during root morphogenesis. These proteins, which make up the majority of the enamel, are 90% amelogenins and the remaining 10% tuftelins and other types of proteins. Subsequently, it was hypothesised that the surgical application of EMD on teeth affected by periodontitis could lead to the formation of a natural extracellular matrix that controlled regenerative processes, mimicking biological interactions. In fact, in vitro studies have shown how EMD has a role in attachment, mitogenesis, biosynthesis and cell differentiation. Furthermore, different cell types have different responses to EMD: it can improve the attachment of periodontal ligament fibroblasts but has no effect on gingival fibroblasts and epithelial cells, which indicates a selective behaviour, actually particularly advantageous in the early stages of healing. Besides, in cultures of periodontal ligament cells, EMD induced an autocrine release of inflammation mediators and soluble factors, including TGF-β1 and IL-6 (Lyngstadaas et al., 2001). Furthermore, EMD can act directly on supragingival plaque, inhibiting the growth of periodontopathogenic bacteria (Arweiler, Auschill, Donos, & Sculean, 2002). Finally, EMD treatment increases the amount of granulation tissue and accelerates the epithelialisation process, thereby also accelerating wound healing, probably due to angiogenic effects and increased levels of growth factors and proteinases. In summary, EMD has been shown to have anti-inflammatory properties and can promote both fibroblast proliferation on the root surface and osteoblast differentiation, being able to promote periodontal regeneration when applied to the root surface in addition to periodontal therapy. The root surfaces of the defects that are treated with EMD are prepared first with scaling and root planing and then conditioned with etching, then removes the smear layer and allows EMD to precipitate on the surface devoid of organic remains.
The clinical and radiographic efficacy of EMD in terms of attachment and bone gain was first demonstrated by two multicentric studies conducted in the Scandinavian countries (Heijl, Heden, Svärdström, & Ostgren, 1997; Zetterström et al., 1997). Subsequently, case reports from various researchers and clinicians confirmed the initial results and established that the application of EMD in intrabony defects promotes a significant gain in clinical attachment (CAL), reduction in probing depth (PD) and bone regeneration. The next step was to directly compare the EMD Guided Regeneration with other regenerative and conventional therapeutic methods, and also with the use of combined techniques. Numerous studies have verified the clinical superiority of the EGR on the technique of the Open Flap Debridement (OFD). The clinical results proved to be equivalent in studies that compared EMD to other regenerative procedures that included a series of resorbable or non-resorbable membranes.
Considering similar costs and equivalent therapeutic outcomes, it seems appropriate to compare EGR and GTR. It is known that the successful application of a membrane depends significantly on the technique used and requires a total coverage of the membrane. Moreover, the shaping and adaptation of the membrane to the root takes time, especially in the posterior sectors, and finally, a suture is also required that holds the membrane in place, a suture that in the case of non-resorbable threads must be removed in a second surgical time. On the contrary, the EMD application is much easier and faster to perform: with a syringe with EMD-containing gel, it is possible to treat multiple defects in the same patient, resulting in a considerable saving of time and costs, both for the patient and the operator.
Systemic benefits and clinical benefits
The clinical trial on which the dissertation is based is the first that compares, in addition to clinical parameters, the acute phase response following non-surgical periodontal treatment with and without the application of enamel matrix derivative.
The data of the present study shows that non-surgical periodontal therapy, performed intensively, with and without the addition of EMD, is able to produce a significant improvement in the clinical periodontal parameters at 3 months and a significant perturbation of systemic inflammation.
Several studies have shown the ability of EMD to promote periodontal regeneration, however, in some situations this potential is limited due to the inability of the EMD gel to support soft tissue in non-containing defects, a key element in order to obtain a predictable regeneration. In recent years, non-surgical periodontal therapy has been increasingly used, thanks to reduced costs and the possibility of being performed not only by specialists. EMD itself could represent an additional tool to add to non-surgical periodontal therapy thanks also to its ability to reduce plaque and the growth of periodontopathogenic bacteria. A study published in 2009 (Mellonig et al. 2009) showed that in 3 of 4 patients treated with non-surgical and EMD therapy, periodontal regeneration was recorded, with the formation of new cement, bone, periodontal ligament and connective tissue attachment. The efficacy of EMD in addition to non-surgical periodontal therapy in promoting regeneration of deep infrabony defects has also been demonstrated in a randomized clinical trial (Aimetti et al. 2017).
In the trial on which the dissertation is based, general success of the administered therapy was reported, and it was possible to register an effective superiority of the addition of EMD to the ultrasonic instrumentation performed in a single session, having had a statistically significant reduction of the pockets> 6mm at 3 months only in the test group.
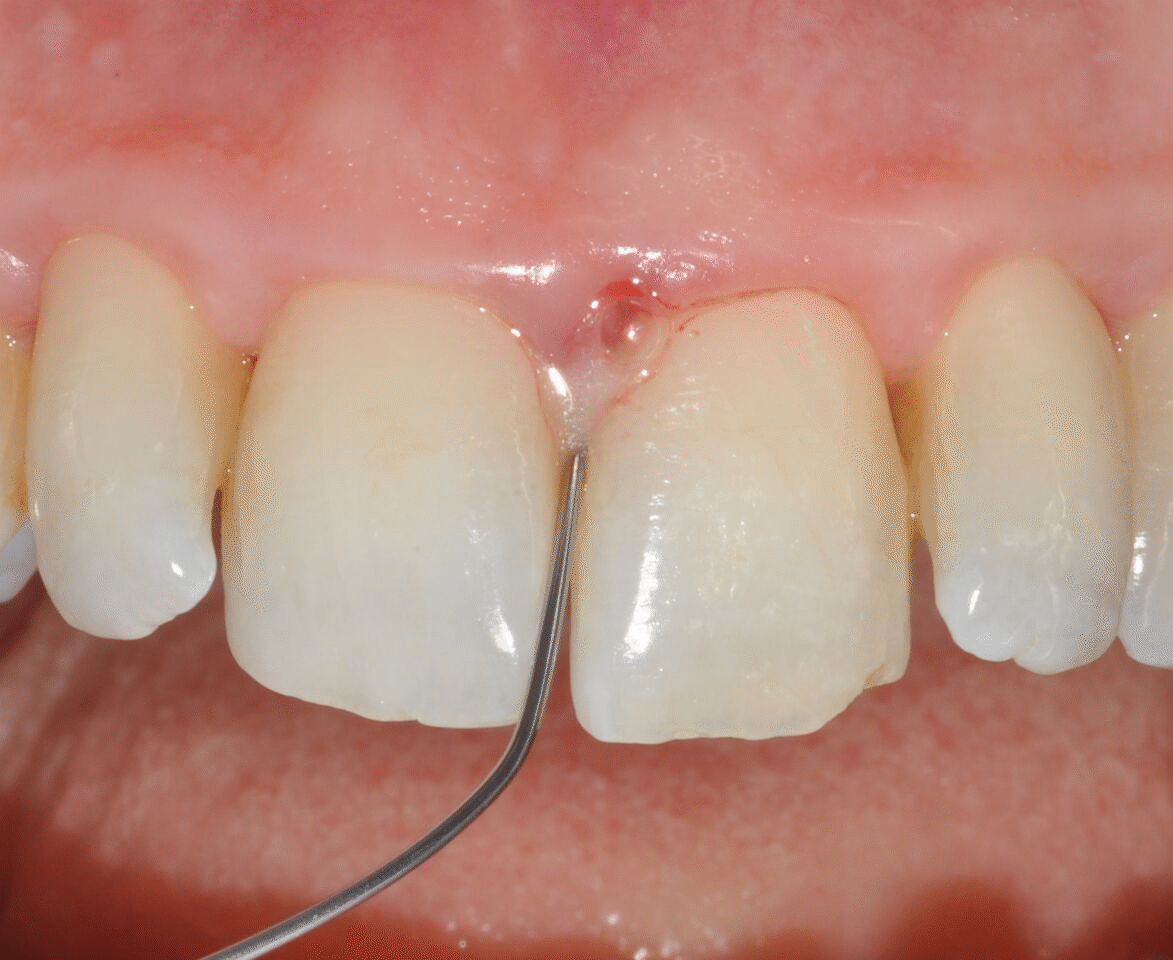
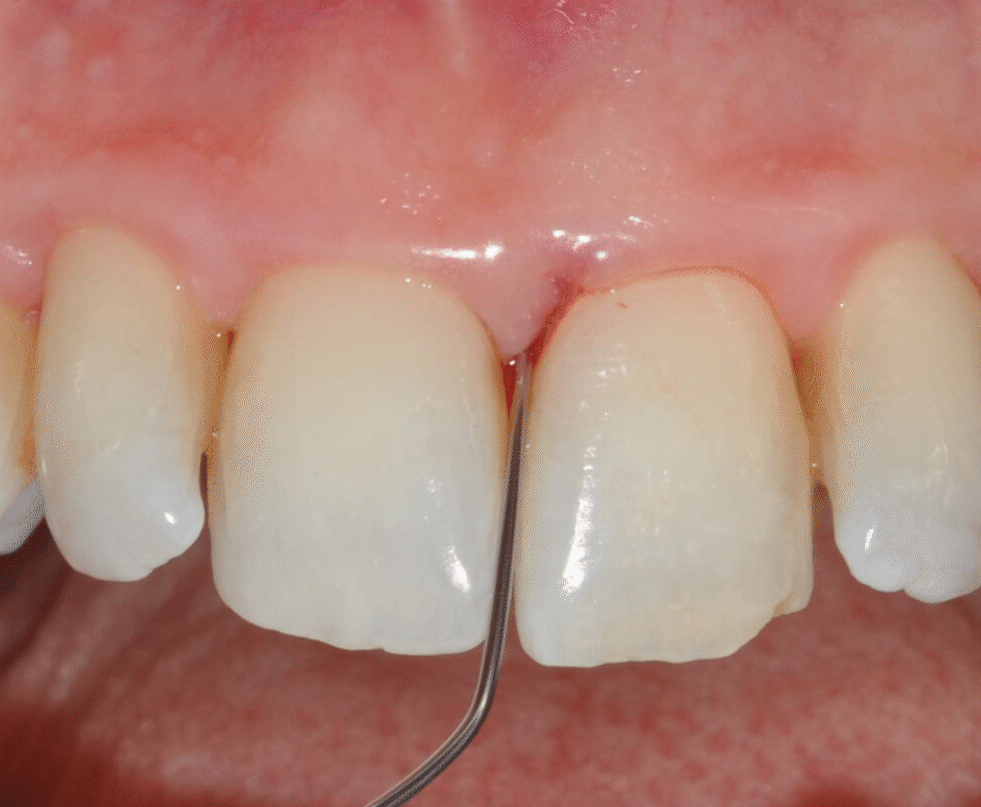
Application of EDTA and Enamel Matrix Derivative gel in the mesial pocket of an upper right central incisor
Regarding the data of systemic markers, an inflammation peak was recorded in both the test and the control group, recorded through significant increases in PCR at 24 hours after treatment. In the test group, an increase in fibrinogen was also found after 24 hours, while in the control group, an increase in D-dimer and Cystatin C was always detected 24 hours after the treatment. These values then returned to values similar to the baseline, although generally slightly decreased. The data produced by this study is in agreement with previous clinical researches that have investigated the effect of non-surgical periodontal therapy on systemic inflammation (D’Aiuto et al. 2013, Graziani et al. 2015), where a moderate acute phase response with an increase in hepatic inflammatory proteins such as CRP following non-surgical periodontal therapy has been consistently reported. The reason for this type of response can be explained by bacteremia and the immune response that follows the damage of local tissues caused by gingival instrumentation, which can lead to an increase in pro-inflammatory markers and from acute-phase proteins. The systemic data relating to the group treated with EMD, are in agreement with the review published by Miron et al. in 2016 (Miron et al. 2016), which demonstrated how this substance could influence the inflammatory response.
The higher levels of CRP at 24 hours do not surprise, because transient inflammation is a fundamental factor for gingival and periodontal healing, allowing the recruitment, differentiation and mobilisation of cells which are fundamental for regeneration. It is interesting to observe the concentration of D-dimer, one of the products of the degradation of fibrinogen, which is particularly increased in subjects with chronic diseases and generally decreases following periodontal treatment: the 24-hour D-dimer data confirms that in the control group systemic inflammation has increased significantly, both in terms of intra-group differences (compared to baseline and 24 h) and intergroup differences (compared to the control group).
A similar but opposite trend was followed by fibrinogen: in the test group, a statistically significant peak was recorded at 24 h and a drastic decrease at 3 months, in accordance with the improvement of clinical parameters. It has been shown that this polymer is released during the dissolution phases of the fibrin clot, which is fundamental for regeneration processes. During coagulation, fibrinogen is converted into fibrin by thrombin, and then this is degraded, causing an increase in degradation products, including D-dimer.
Considering that in the 24-hour test group the fibrinogen is characterised by a statistically significant peak, but one of its last degradation products, the D-dimer, seems to remain constant, it is possible to hypothesise that the presence of EMD may delay the degradation of the clot of fibrin, compared to the control groups.
Innovation
The results show how the addition of EMD to Fm-UD is associated with a lower acute-phase inflammatory response compared to Fm-UD alone. Furthermore, Fm-UD + EMD has been shown to be more effective in reducing probing depth than in control at 3 months from therapy, reducing the need for additional therapies by 50%. This could be due to some possible anti-inflammatory effects of EMD.
The addition of EMD for non-surgical periodontal treatment in patients with a complicated medical history and/or comorbidities where there is a need to control systemic inflammatory status could be considered, furthermore in the periodontal sites with a more advanced pathology status it could be the addition of EMD to ultrasonic periodontal instrumentation is recommended.
Literature and suggested reads:
Lyngstadaas, S. P., Lundberg, E., Ekdahl, H., Andersson, C., & Gestrelius, S. (2001). Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. Journal of Clinical Periodontology, 28(2), 181–8. https://www.ncbi.nlm.nih.gov/pubmed/11168744
Arweiler, N. B., Auschill, T. M., Donos, N., & Sculean, A. (2002). Antibacterial effect of an enamel matrix protein derivative on in vivo dental biofilm vitality. Clinical Oral Investigations, 6(4), 205–9. https://www.ncbi.nlm.nih.gov/pubmed/12483234
Heijl, L., Heden, G., Svärdström, G., & Ostgren, A. (1997). Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. Journal of Clinical Periodontology, 24(9 Pt 2), 705–14. https://www.ncbi.nlm.nih.gov/pubmed/9310876
Zetterström, O., Andersson, C., Eriksson, L., Fredriksson, A., Friskopp, J., Heden, G., … Gestrelius, S. (1997). Clinical safety of enamel matrix derivative (EMDOGAIN) in the treatment of periodontal defects. Journal of Clinical Periodontology, 24(9 Pt 2), 697–704. https://www.ncbi.nlm.nih.gov/pubmed/9310875
Mellonig, J.T., Valderrama, P., Gregory, H.J., Cochran, D.,L. (2009). Clinical and histologic evaluation of non-surgical periodontal therapy with enamel matrix derivative: a report of four cases. Journal of Periodontology, Sep;80(9):1534-40. https://www.ncbi.nlm.nih.gov/pubmed/19722806
Aimetti, M., Ferrarotti, F., Mariani, G.M., Romano, F. (2017) A novel flapless approach versus minimally invasive surgery in periodontal regeneration with enamel matrix derivative proteins: a 24-month randomized controlled clinical trial. Clinical Oral Investigations, 2017 Jan;21(1):327-337. https://www.ncbi.nlm.nih.gov/pubmed/27044318
D’Aiuto, F., Orlandi, M., & Gunsolley, J. C. (2013). Evidence that periodontal treatment improves biomarkers and CVD outcomes. Journal of Clinical Periodontology, 40, S85–S105. https://www.ncbi.nlm.nih.gov/pubmed/23631587
Graziani, F., Cei, S., Orlandi, M., Gennai, S., Gabriele, M., Filice, N., … D’Aiuto, F. (2015). Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: A randomized clinical trial. Journal of Clinical Periodontology, 42(9), 843–52. https://www.ncbi.nlm.nih.gov/pubmed/26309133
Miron, R.J., Sculean, A., Cochran, D.L., … (2016). Twenty years of enamel matrix derivative: the past, the present and the future. Journal of Clinical Periodontology, Aug;43(8):668-83. https://www.ncbi.nlm.nih.gov/pubmed/26987551
