A challenging case - December 10, 2021
By Andrea Roccuzzo1,2, presented by Mario Romandini3
1 Department of Periodontology, School of Dental Medicine, University of Bern, Bern, Switzerland
2 Department of Oral and Maxillofacial Surgery, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark
3 ETEP (Etiology and Therapy of Periodontal and Peri-implant Diseases) Research Group, University Complutense, Madrid, Spain
ABSTRACT
Peri-implantitis represents the most considerable concern for many dental clinicians nowadays due to its detrimental effect on implant longevity. Due to the limited efficacy of the non-surgical approaches, several surgical interventions have been proposed.
The present report describes the surgical treatment of an implant placed in the posterior mandible affected by peri-implantitis. After flap elevation, soft-tissue degranulation and implant surface decontamination, the intrabony defect was filled using DBBM-C. Following healing, the patient was enrolled in an individualized supportive periodontal/peri-implant (SPT) program and was followed up to 1 year.
This case presentation reports on all the diagnostic and surgical aspects, step-by-step, providing the reader with an overview of this particular treatment modality.
In this issue's PerioArena, I have the privilege to introduce a case by Dr. Andrea Roccuzzo from the University of Bern. Most of you already know Andrea for his important scientific contributions in the last few years. Despite his young age, he already contributed to the implant- and perio-World with researches published in some of the highest impacted dental journals. Moreover, he is also an elegant clinician, which you will surely appreciate looking at this case report.
To me, before being a colleague, Andrea is a true friend. We met long before deciding to further our education in Periodontology, as two undergraduate students in Dentistry, at an event of the Italian Society of Dental Students in Rome. From the beginning, we felt as if we would have been friends all of our lives. One of Andrea's features? He is a true gentleman. [And, of course, he is a Juventus fan (which may be becoming an inclusion criterion for being invited to the PerioArena…)]
In this PerioArena, Andrea will present the (equally) elegant regenerative surgery of a peri-implantitis case he treated in Bern. At the end of his case report, you will find – as always - a brief "Question and Answer" section, giving better insight into some specific technical details of this particular case.
It's time to give the floor to Andrea for presenting his case!
Mario Romandini
1. Case presentation and management
A 65-year old male patient was referred in February 2020 to the Department of Periodontology of the University of Bern, Switzerland by his private dentist. He had been rehabilitated in July 2009 with one tissue level implant (Institut Straumann AG, Basel Switzerland) in region 36, supporting a single-unit cemented fixed dental crown (SUC). The metal-ceramic SUC had been cemented permanently with a glass ionomer cement (3M™ ESPE Ketac™ Cem, Seefeld, Germany). Unfortunately, implant 36 had been diagnosed with peri-implantitis by the referring dentist in the course of regular supportive therapy.
The patient was in good general health, non-smoker and exhibited a good level of self-performed plaque control.
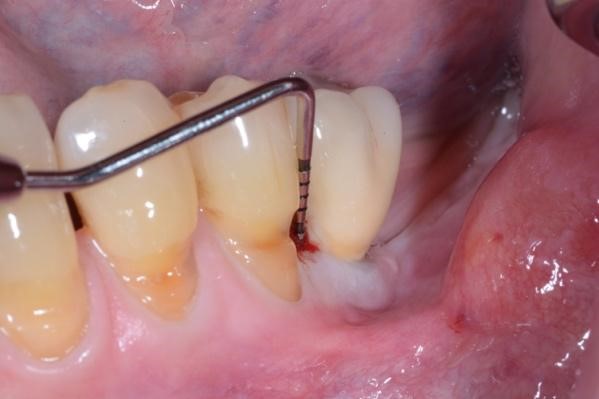
At the clinical examination, signs of peri-implantitis (i.e. increased pocket probing depth with concomitant bleeding on probing and suppuration) associated with radiographic signs of progressive peri-implant marginal bone loss (compared to the 1-year post-loading radiograph) on the mesial aspect of the implant were detected (Figure 1-2). The implant-supported SUC had been cemented permanently and could not be removed. The patient's chief complaint was to treat the affected implant but without removing the reconstruction for financial reasons.
2. Pre-surgical phase
Oral hygiene instructions were provided and reinforced 8 weeks before the surgical intervention. In addition, a single session of non-surgical therapy was carried out consisting in peri-implant surfaces debridement under local anesthesia using carbon fiber curettes. After mechanical debridement, the pockets around the implants were rinsed with sterile saline solution.
3. Surgical procedure
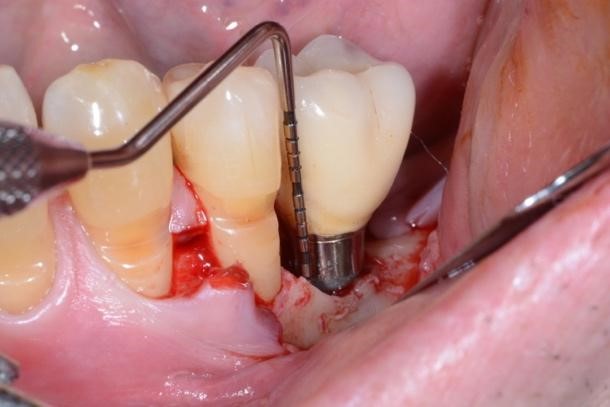
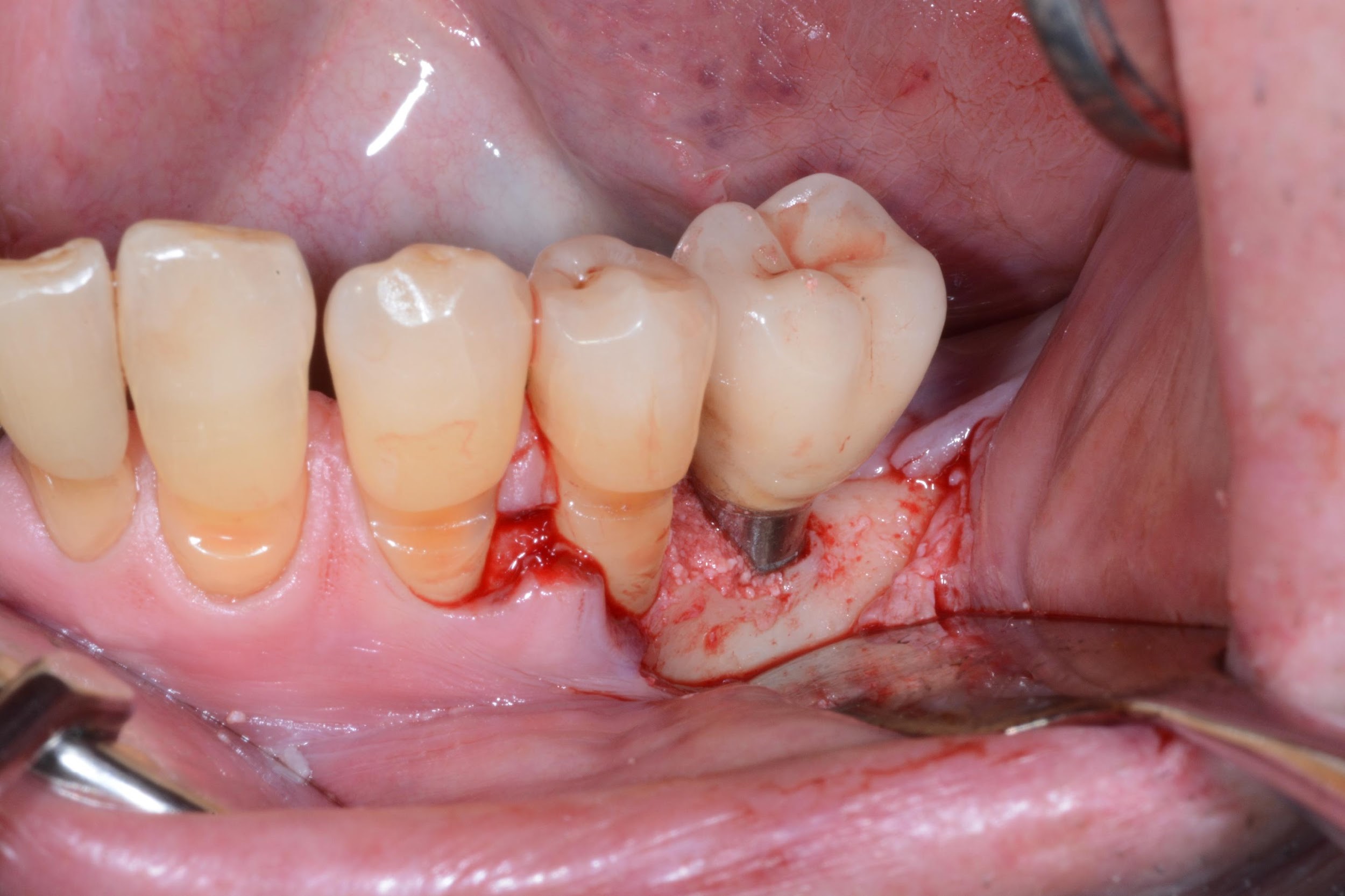
Under local anesthesia, access to the peri-implant infected surface was gained by means of a full-thickness flap. In order to avoid vertical releasing incisions but to allow optimal surgical access to the peri-implant defect, a papilla base incision between teeth 34 and 35 was performed, followed by an intrasulcular incision which was extended distally to the region of the implant. Consequently, after flap elevation only on the buccal aspect of the defect, all the granulation tissue was carefully removed with dedicated titanium curettes and implant surface decontamination was implemented using a Chitosan Brush under irrigation alternatively of chlorhexidine digluconate and saline solutions. Thereafter, a deproteinized bovine bone mineral with 10% collagen (DBBM-C) (Bio-Oss Collagen, Geistlich, Wolhusen, Switzerland), moistened in sterile saline, was applied to fill the 3-wall intrabony defect. Finally, the flap was repositioned coronally and fixed with sutures to ensure a non-submerged healing procedure (Figure 2-3).
4. Post-surgical care
The patient was prescribed to take 1 g of amoxicillin and clavulanic acid twice a day for 6 days, starting 1 h prior to surgery, and non-steroidal analgesics, as needed. Immediately after surgery, the patient applied ice packs at the treated area, and it was recommended that they keep in place for at least 4 h. Patient were advised to discontinue tooth brushing and to avoid trauma at the site of surgery for 3 weeks. He was also instructed to use 0.2% chlorhexidine digluconate rinse for 1 min three times a day for the same period of time. The patient was seen after 7 days and then weekly for the first month to monitor healing. The sutures were removed after 14 days. After the healing phase, the patient was placed on an individually tailored SPT program with a recall interval of 3 months for the first year.
5. 1-year follow-up
6. Discussion
During the last decade, the scientific interest in the surgical treatment of peri-implantitis, especially due to the overall limited efficacy of the non-surgical approaches (Roccuzzo et al. 2020), has dramatically increased, as shown by the high number of published publications (Roccuzzo et al. 2021). Nevertheless, at present, no definitive conclusion can be drawn on which should be considered the "best surgical protocol" (Tomasi et al. 2019). The presented surgical protocol aimed to recreate the conditions that favor re-osseointegration and limit the post-operative soft tissue recession. For this purpose, a full-thickness flap raised only on the buccal side, to gain access to the contaminated implant surface has been performed. Thereafter, careful peri-implant soft tissue degranulation using dedicated Ti-curettes and decontamination using a Chitosan Brush was performed. One of the still open questions is whether submerged healing should be preferable after surgical reconstructive interventions. This topic has been recently investigated in a 12-month prospective case series on 15 patients rehabilitated with 27 dental implants by Monje and co-authors (Monje et al. 2020). The advantage of this approach would be to achieve primary wound closure and promote aseptic healing.
On the other hand, the applied protocol implied non-submerged healing mainly to decrease the post-operative discomfort and the overall complexity and treatment time. Nevertheless, irrespective of the healing modalities, the importance of creating a thick peri-implant soft tissue seal has been widely underlined in the literature as a crucial aspect of achieving treatment success (Roccuzzo et al. 2017, 2020; Monje et al. 2020). Therefore, clinicians should carefully evaluate the quality of the peri-implant soft tissues before surgical reconstructive interventions.
When focusing on the grafting material used, a DBBM-C was chosen mainly due to its better handling properties (i.e. optimal adhesion to the site, tailoring to the morphology of the defect, long-term graft stability due to the low resorption rate) compared to other material granules (Araújo et al. 2010; Sculean et al. 2005; Mercado et al. 2018).
Besides the surgical details which might have an impact on the short-term outcomes, the increasing evidence on the long-term (i.e. > 5-year follow-up) efficacy of peri-implantitis surgical interventions whether by resective (Berglundh et al. 2018, Heitz-Mayfield et al. 2018) or reconstructive (Roccuzzo et al. 2017; Isehed et al. 2018) approaches, stressed the importance of patients' enrollment and adhesion to a tailored SPT program to maintain the positive short-term results (Roccuzzo et al. 2018). In the presented case, the patient exhibited optimal plaque and bleeding scores during the whole observation period up to 12 months, which might indirectly prove their importance to maintain stable results.
7. Conclusions
Peri-implantitis is not a rare finding in the population. Therefore, early correct diagnosis is crucial to treat this pathological condition successfully. The present case report depicts the surgical treatment of an implant affected by peri-implantitis with a reconstructive non-submerged approach. One year after treatment, complete disease resolution was observed associated with the patient's satisfaction.
Conflict of interest: The authors do not have any financial interests, either directly or indirectly, in the products or information presented within the paper. A.R. is the recipient of a 3-year scholarship from the Clinical Research Foundation (CFR) for the Promotion of Oral Health, Brienz, Switzerland.
"Question & Answer" section
1. Assuming a meticulous implant surface decontamination, do you think in contained intrabony defects, the use of a biomaterial is always mandatory?
The rationale behind the application of a biomaterial in an intrabony peri-implant defect is drawn from the periodontal surgery: indeed, it has been widely demonstrated that in cases of infra-bony defects at tooth site, the application of biomaterials results in better clinical outcomes (i.e. CAL gain and PPD reduction) than an open-flap debridement procedure enhancing in some cases periodontal regeneration. Consequently, it seems plausible that applying a bone graft within a peri-implant defect might favour the healing, limiting the risk for the flap to collapse and enhancing re-osseointegration, which is the ultimate goal of a reconstructive procedure.
2. Could you share your criteria for deciding when to add a membrane and perform submerged healing when carrying out regenerative surgery of peri-implantitis cases?
The decision of performing submerged healing is mainly related to three aspects:
1. Presence of an intrabony peri-implant defect with regenerative potential
2. Presence of a screw-retained reconstruction which allows after the healing phase a prosthesis reconnection
3. Presence of at least 2 mm of peri-implant keratinized mucosa (KT)
If all these conditions are satisfied and the patient accepts to stay at least 8 weeks without the prosthesis, submerged healing might be a reliable treatment option. In this case, the use of a barrier membrane to stabilize the graft particles and avoid the epithelial cells in-growth can be used. Nevertheless, it has to be underlined that at present, there is no evidence on the superiority of a reconstructive treatment with the application of a barrier membrane.
Figure 1. A) Clinical buccal view of the inflamed peri-implant mucosa at site 36 during the first clinical examination. The patient did not report any pain in this particular area. Pocket probing depths (PPD) up to 8 mm with concomitant bleeding on probing (BoP) and suppuration were detected. B) The 2D radiographic image reveals the presence of peri-implant marginal bone resorption on the mesial peri-implant surface.
 |
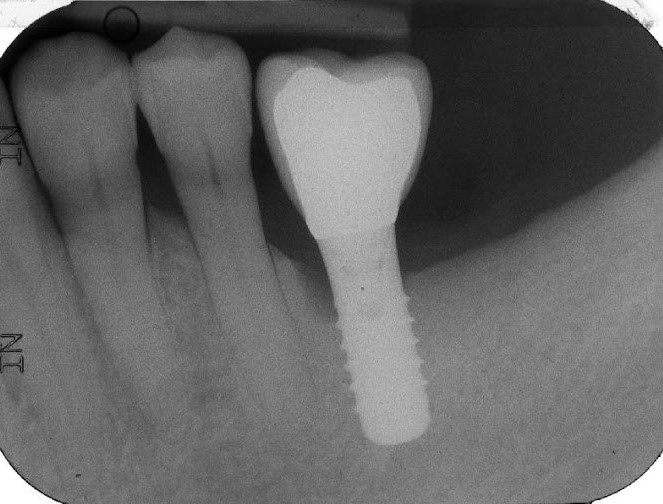 |
Figure 2. A) Clinical intraoperative view of the peri-implant defect (i.e. 3 mm) after soft tissue degranulation. B) Peri-implant surface decontamination performed with a dedicated Chitosan Brush (Labrida BioClean™ - Straumann)
 |
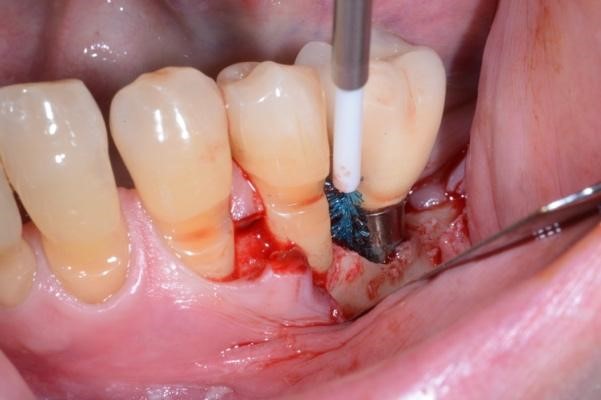 |
Figure 3. A) Clinical view of the peri-implant defect filled with a DBBM-C B) Clinical appearance of the treated area after suturing. Note the non-submerged healing.
|
 |
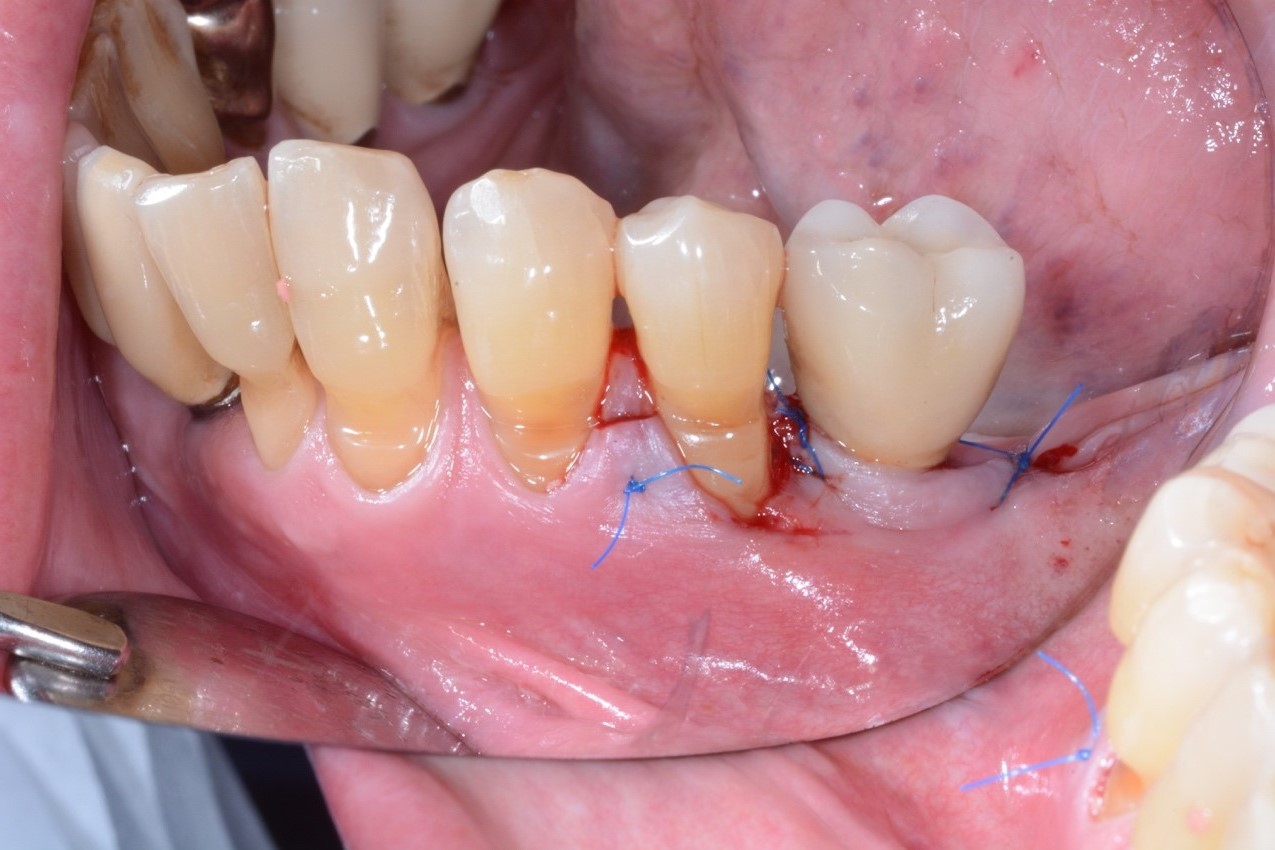 |
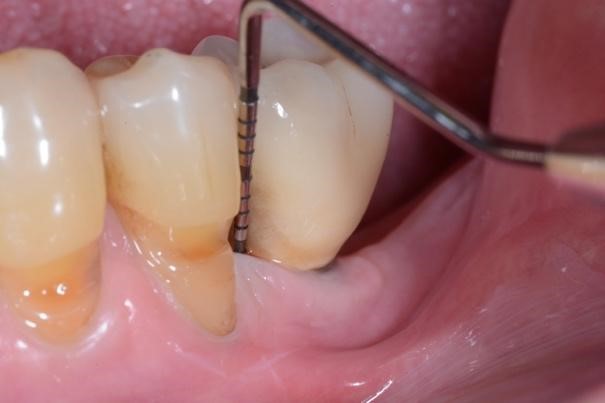
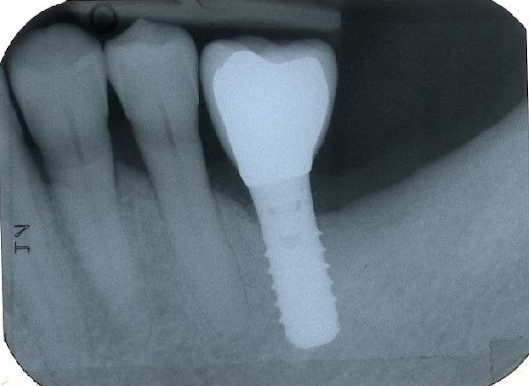
Figure 4. A) Clinical buccal view of the inflamed peri-implant mucosa at site 36 at the 1-year clinical examination. Pocket probing depths (PPD) up to 4 mm with no concomitant bleeding on probing (BoP) and suppuration were detected. B) The 2D radiographic image reveals substantial filling of the peri-implant bone defect.
|
|
Literature and suggested reads
- Tomasi, C., Regidor, E., Ortiz-Vigón, A., & Derks, J. (2019). Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. Journal of Clinical Periodontology, 46(21), 340-356. http://doi.org/10.1111/jcpe.13070.
- Roccuzzo, A., De Ry, S. P., Sculean, A., Roccuzzo, M., Salvi, G. E. (2020). Current Approaches for the Non-surgical Management of Peri-implant Diseases. Current Oral Health Reports, 7(3), pp. 274–282.
- Roccuzzo, A.; Stähli, A.; Monje, A.; Sculean, A.; Salvi, G. E. (2021). Peri-Implantitis: A Clinical Update on Prevalence and Surgical Treatment Outcomes. Journal of Clinical Medicine, 10, 1107. https://doi.org/10.3390/ jcm10051107
- Monje, A., Pons, R., Roccuzzo, A., Salvi, G. E., Nart, J. (2020). Reconstructive therapy for the management of peri-implantitis via submerged guided bone regeneration: A prospective case series. Clinical Implant Dentistry and Related Research, 22(3), 342-350. https://doi.org/10.1111/cid.12913
- Roccuzzo, M., Pittoni, D., Roccuzzo, A., Charrier, L., & Dalmasso, P. (2017). Surgical treatment of peri-implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7-year-results. Clinical Oral Implants Research, 28(12), 1577–1583. https://doi. org/10.1111/clr.13028
- Roccuzzo, M., Fierravanti, L., Pittoni, D., Dalmasso, P., Roccuzzo A. (2020). Implant survival after surgical treatment of peri-implantitis lesions by means of deproteinized bovine bone mineral with 10% collagen: 10-year results from a prospective study. Clinical Oral Implant Research, 31, 768–776. https://doi.org/ 10.1111/clr.13628
- Araújo, M. G., Liljenberg, B., Lindhe, J. (2010). Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clinical Oral Implants Research, 21(1), 55-64. https://doi.org/10.1111/j.1600-0501.2009.01854.x
- Sculean, A., Chiantella, G. C., Windisch, P., Arweiler, N. B., Brecx, M., Gera, I. (2005). Healing of intra-bony defects following treatment with a composite bovine-derived xenograft (Bio-Oss Collagen) in combination with a collagen membrane (Bio-Gide PERIO). Journal of Clinical Periodontology, 32(7), 720-4. https://doi.org/10.1111/j.1600-051X.2005.00758.x
- Mercado, F., Hamlet, S., Ivanovski, S. (2018). Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and Doxycycline-A prospective 3-year cohort study. Clinical Oral Implants Research, 29(6), 583-591. https://doi.org/10.1111/clr.13256.
- Berglundh, T., Wennström, J. L., & Lindhe, J. (2018). Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clinical Oral Implants Research, 29(4), 404-410. https://doi.org/10.1186/s40729-017-0106-210.1111/clr.13138
- Heitz-Mayfield, L. J. A., Salvi, G. E., Mombelli, A., Loup, P. J., Heitz, F., Kruger, E., & Lang, N. P. (2018). Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clinical Oral Implants Research, 29(1), 1-6. https://doi.org/10.1111/clr.12910.
- Isehed, C., Svenson, B., Lundberg, P., & Holmlund, A. (2018). Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: 3-and 5-year follow-up. Journal of Clinical Periodontolology, 45, 744–753. https://doi.org/10.1111/jcpe.12894
- Roccuzzo, M., Layton, D. M., Roccuzzo, A., & Heitz-Mayfield, L. J. (2018). Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review. Clinical Oral Implants Research. 29(16), 331-350. https://doi.org/10.1111/clr.13287
